Recombinant albumin is a key ingredient for vaccine manufacturing, but its price and limited availability have put certain animal-free vaccines out of reach for many low and middle-income countries (LMIC).
This case study describes how Phenotypeca, with support from the Bill & Melinda Gates Foundation, set out to develop optimised strains of baker’s yeast to enable the production of recombinant human albumin at commercial scale and lower cost.
Widening access to high quality, animal-free vaccines is a potential lifesaver for LMIC populations. This was a strong motivator for the whole team.
Challenge
Existing suppliers of recombinant albumin keep their know-how of the manufacturing process tightly protected. This acts as a barrier to entry against competitors. enabling incumbents to sell their limited output to premium markets at high prices.
The challenge, therefore, was to find a new and more cost-effective way of manufacturing recombinant albumin to equivalent standards – at a large scale.
Solution
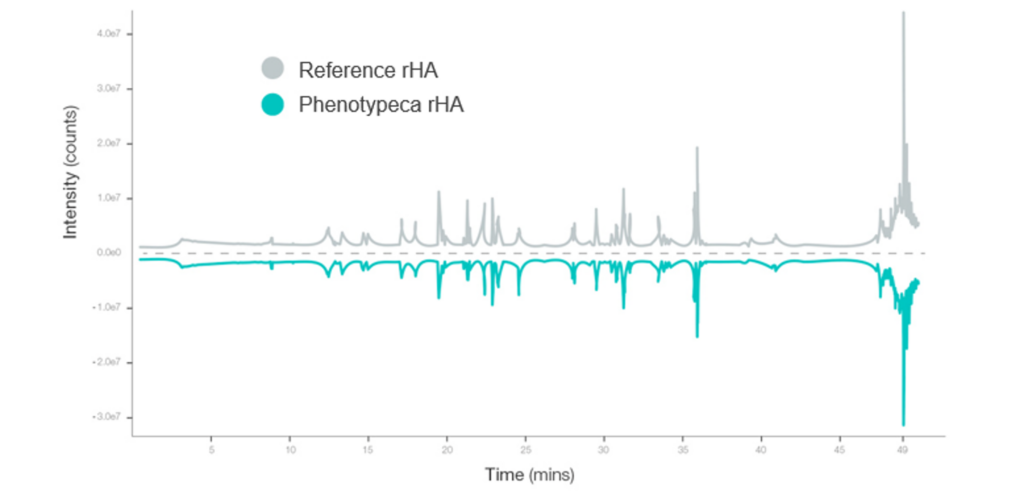
Phenotypeca has used proprietary Quantitative Trait Loci (QTL) technology to optimise strains of Saccharomyces cerevisiae. These yeast strains produce high-quality albumin and are better adapted to manufacturing at a commercial scale for developing countries.
Key outcome
In its first phase, the new approach has already delivered a 5-10 fold increase in yield (titre), enabling supply costs to drop significantly.
What’s next?
QTL technology promises to transform recombinant protein manufacturing across a range of applications. Our optimised strains have already delivered an outstanding 10-20 fold increase in titre for VHH antibodies compared to traditional methods.
In the hands of Phenotypeca’s experienced team, QTL technology can optimise the development and production of biosimilars, proprietary biologics and cosmeceuticals in a way that’s tailored to the requirements of their markets – while also enhancing patent protection.
Download the case study here – Albumin Interim Case Study

